| Peter Faller |
| Transporting Cu into Cells with a Peptide |
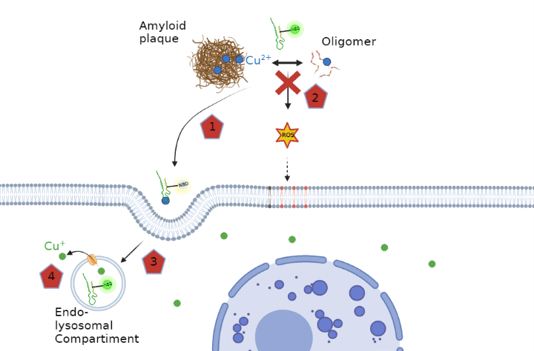
In Alzheimer’s disease (AD) copper ions accumulates in amyloid plaques, a hallmark of this disease. This copper likely originating from intracellular sources. The Cu in amyloid plaques is bound to its main constituent, the peptide amyloid-β (Aβ). Cu-Aβ can catalyze the production of reactive oxygen species (ROS) in vitro and is thought to contribute to the oxidative stress observed in AD. The paper reports on the development of a novel Cu shuttle able to restore the Cu balance between intra- and extracellular Cu. The shuttle has a peptide scaffold and consists of two units, a selective Cu(II)-chelation part and a cell penetrating part. Moreover, the shuttle was tagged with a Cu(II)-binding sensitive fluorophore to monitor the localization and Cu(II)-binding state. This shuttle coordinates the Cu(II) in a highly selective manner, is able to remove Cu from Aβ, to stop ROS production of Cu-Aβ, and to transport Cu(II) into the cell, where Cu(II) is reduced, and Cu(I) is released in a bioavailable form. This shuttle could be a valuable new tool for basic research and has potential applications in the restoration of Cu homeostasis, such as observed in AD.
Title of the article: Development of a Cu(II)-specific peptide shuttles capable of preventing Cu-Amyloid beta toxicity and importing bioavailable Cu into cells
List of authors: Michael Okafor, Paulina Gonzalez, Pascale Ronot, Islah El Masoudi, Anne Boos, Stéphane Ory, Sylvette Chasserot-Golaz, Stéphane Gasman, Laurent Raibaut, Christelle Hureau, Nicolas Vitale, Peter Faller
Citation: Chem. Sci. 2022, 13, 11829-11840
Url: https://pubs.rsc.org/en/Content/ArticleLanding/2022/SC/D2SC02593K
Laboratory: Institut de Chimie, UMR 7177
City: Strasbourg
Country: France